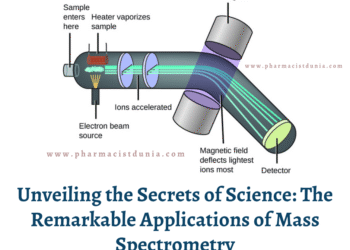
Impurities
An impurity in a drug substance as defined by the international conference on harmonization (ICH) guidelines is -any component of the drug substance that is not the chemical entity defined as the drug substance and affects the purity of active ingredient or drug substances.
An impurity in a drug product is any component of the drug that is not the chemical entity defined as the drug substance or an excipient in the drug product.
Common terms of impurities:
1. Intermediate penultimate intermediate andn byproducts
2. Transformation products
3. Interaction products
4. Related products
5. Degradation products
Classification of impurities:
Impurities in drug substance can be classified into the following categories-
1. Organic impurities
2. Inorganic impurities
3. Residual solvents
Sources of impurities:
1. Raw materials
2. Method of manufacture
3. Impurities due to storage condition
a) Chemical instability
b) Changes in the physical properties
c) Reaction with container materials
d) Temperature